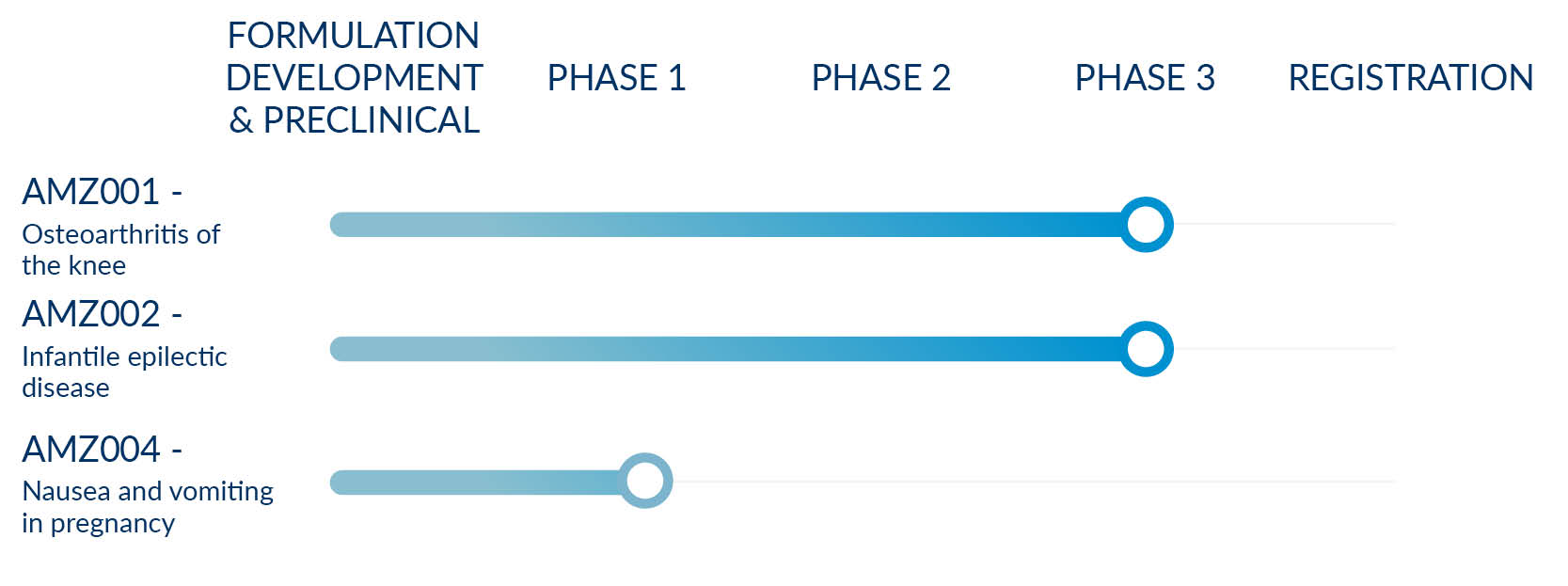
Pipeline
We are already involved in three programs, with others under discussion.
Click + to expand boxes to reveal program details.
AMZ001 - Osteoarthritis of the knee
TOPICAL DICLOFENAC GEL FOR THE TREATMENT OF PAIN RELATED TO KNEE OSTEOARTHRITIS
AMZ001 is a high strength, discrete, odorless and non-invasive topical gel formulation containing 3.06% diclofenac sodium. Applied directly to the knee and designed as an FDA and EMA approved proprietary technology. This non-steroidal anti-inflammatory (NSAID) medication is intended for the treatment of pain related to knee osteoarthritis (OA).
As a topical product, AMZ001 is not absorbed into the gastrointestinal (GI) tract, reducing overall GI and systemic exposure which could help to reduce adverse effects compared to oral therapies.
Its higher drug strength (3.06%) combined with an efficient topical delivery system allows AMZ001 to effectively relieve localized pain with a single daily application (QD) which reduces the total dose compared to other topical NSAIDs treatments.
Indication
AMZ001 has been designed to treat the pain caused by osteoarthritis of the knee, effectively targeting the affected tissues with rapid and sustained analgesia. Osteoarthritis (OA) is the most common type of arthritis and is a major cause of chronic musculoskeletal pain and disability in elderly populations.
OA affects up to 50% of the adult population over the age of 65 with pain being the most significant symptom. Knee osteoarthritis is currently treated with both oral and topical NSAIDs.
Other potential indications for AMZ001 include the management of acute pain due to minor strains, sprains, and contusions, as well as for the treatment of low back pain, which is currently treated using opioids.
Clinical status
Completion of a 21-day, randomized, controlled study to evaluate the skin irritation potential of AMZ001 in 40 healthy volunteers using a cumulative irritation patch test design (CIPT).
Completion of a randomized, controlled study to evaluate the sensitizing potential of AMZ001 in 200 healthy volunteers, using a repeat insult patch test design (RIPT).
Completion of two Phase 1 pharmacokinetic studies: one sequential PK trial including 12 healthy volunteers and one parallel-group PK trial including 75 healthy volunteers.
Successful completion of a four-week Phase 2/3 efficacy study in 440 patients in three different countries: United States, Denmark, and Czech Republic.
More information at:
https://clinicaltrials.gov/ct2/show/NCT03691844
https://clinicaltrials.gov/ct2/show/NCT03691844?term=AMZ001-006&rank=1
https://www.clinicaltrialsregister.eu/ctr-search/search?query=AMZ001-006
Publication in 'Seminars in Arthritis and Rheumatism' Volume 50, Issue 6, December 2020, pg. 1203-1213
Poster presented at OARSI World Congress 2020:

AMZ002 - Infantile epilectic disease
HIGHLY PURIFIED SYNTHETIC HORMONE STERILE INTRAMUSCULAR INJECTION FOR THE TREATMENT OF EPILEPSY
AMZ002 is a sterile injectable hormone product, in-licensed from Ferring. Amzell owns the global rights to the product except in India where Ferring maintains its rights. AMZ002 will offer a highly-purified synthetic alternative to the existing natural FDA-approved product for the treatment of epileptic seizure.
Amzell has conducted an upgrading of the CMC (chemistry, manufacturing and controls) of the product to comply with US regulatory requirements. Amzell has received FDA clearance authorizing clinical testing after submission of the IND (investigational new drug) application.
Clinical status
Phase 1 clinical trial successfully completed.
Amzell BV has concluded discussions with the FDA ahead of initiating a Phase 3 trial for AMZ002. Following the Type B End of Phase 2 meeting with the FDA Division of Neurology, Amzell BV has agreed the study design, dosing, primary and secondary endpoints, and the estimated sample size for the Phase 3 trial. Amzell received concurrence from the FDA using the Special Protocol Assessment (SPA) process.
AMZ004 - Nausea and vomiting in pregnancy
Transdermal gel to prevent NAUSEA AND VOMITING IN PREGNANCY INCLUDING HYPEREMESIS GRAVIDARUM
AMZ004 is an odorless, skin-friendly and convenient transdermal gel formulation of a potent anti-emetic agent. This formulation contains an FDA approved serotonin 5-HT₃ receptor antagonist for the prevention of nausea and/or vomiting associated with emetogenic cancer therapy and for the prevention and treatment of postoperative nausea and vomiting in adults.
Designed for the treatment of severe cases of nausea and vomiting including hyperemesis in pregnancy (an orphan drug designated condition), AMZ004 offers an innovative, patient friendly and better-tolerated route of drug delivery to address this unmet need.
There is currently no pharmacologic treatment approved by the FDA for severe cases of nausea and vomiting in pregnancy including hyperemesis gravidarum. This represents a unique commercial opportunity in an under served market.
Clinical status
Amzell has received regulatory guidance at a pre-IND meeting with the FDA. AMZ004 is eligible for the 505(b)(2) regulatory pathway providing limited regulatory risk, lower cost and shorter development timelines.
AMZ004 has completed the pre-clinical testing required to initiate human clinical studies.
Amzell will initiate the first Phase 1 clinical study of AMZ004 in 2021.
